AP-101: Targeting misfolded SOD1 to halt ALS progression
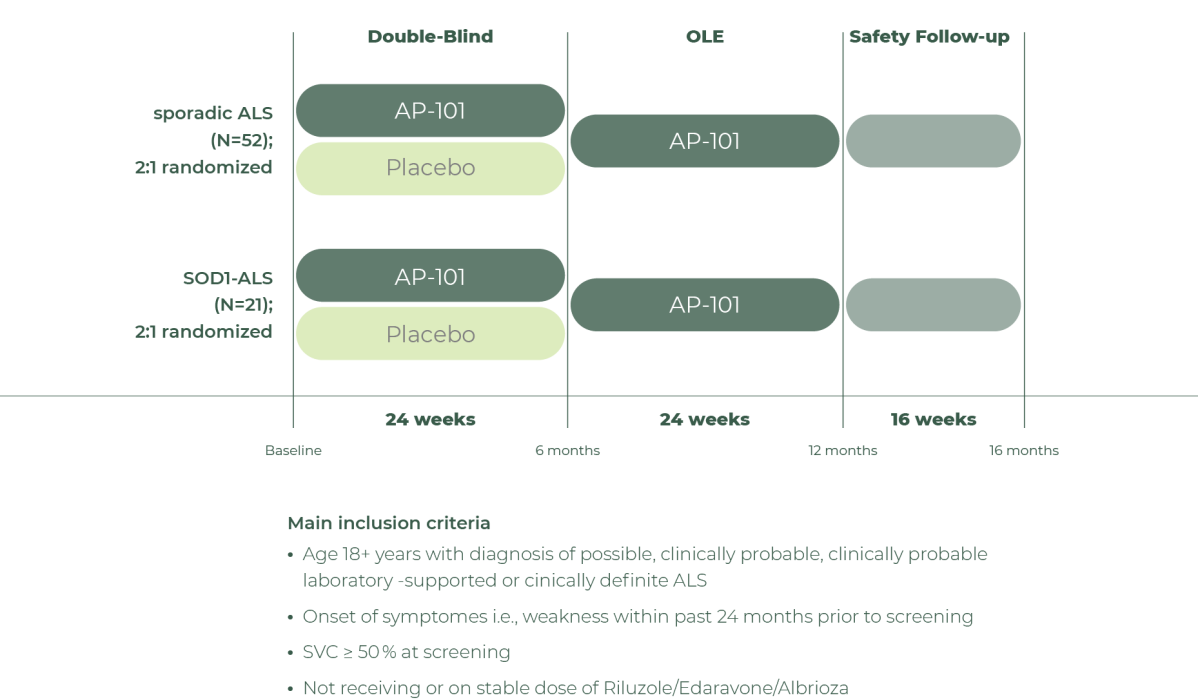
AP-101 progressed through a Phase 1 study initiated in 2019, establishing safety and tolerability in ALS patients using a 3+3 design. The subsequent Phase 2 multicenter, randomized, double-blind, placebo-controlled trial launched in 2021 across Europe, South Korea, Canada, and the U.S., enrolling 73 patients – 52 with sporadic ALS and 21 with SOD1-ALS. The study included a 24-week treatment period followed by a 24-week open-label extension. Primary endpoints were safety and tolerability, with secondary and exploratory endpoints assessing pharmacokinetics, pharmacodynamics, biomarkers – including neurofilament levels in cerebrospinal fluid and serum, as well as various clinical endpoints. The rationale for testing in both populations is based on evidence that misfolded SOD1 contributes to both sporadic ALS and SOD1-ALS. The company presented positive phase 2 results at the 36th International Symposium on ALS/MND.

«As a principal investigator in the AP-101 trial, I’ve seen firsthand the importance of targeting underlying disease mechanisms in ALS. Misfolded SOD1 represents a compelling therapeutic target, and the clinical evaluation of AP-101 marks a meaningful step toward bringing new treatment options to patients.»
Trial Investigator