ALS is a progressive neurodegenerative disease that leads to loss of independence and a shortened lifespan
Amyotrophic lateral sclerosis (ALS) is a relentless and progressive neurodegenerative disease that affects the motor neurons of the brain and spinal cord. Symptoms vary from person to person, some forms of ALS begin with limb weakness, while others start with bulbar symptoms. All forms ultimately lead to loss of independence and a markedly shortened lifespan. Median survival remains three to five years, and diagnosis is often delayed.
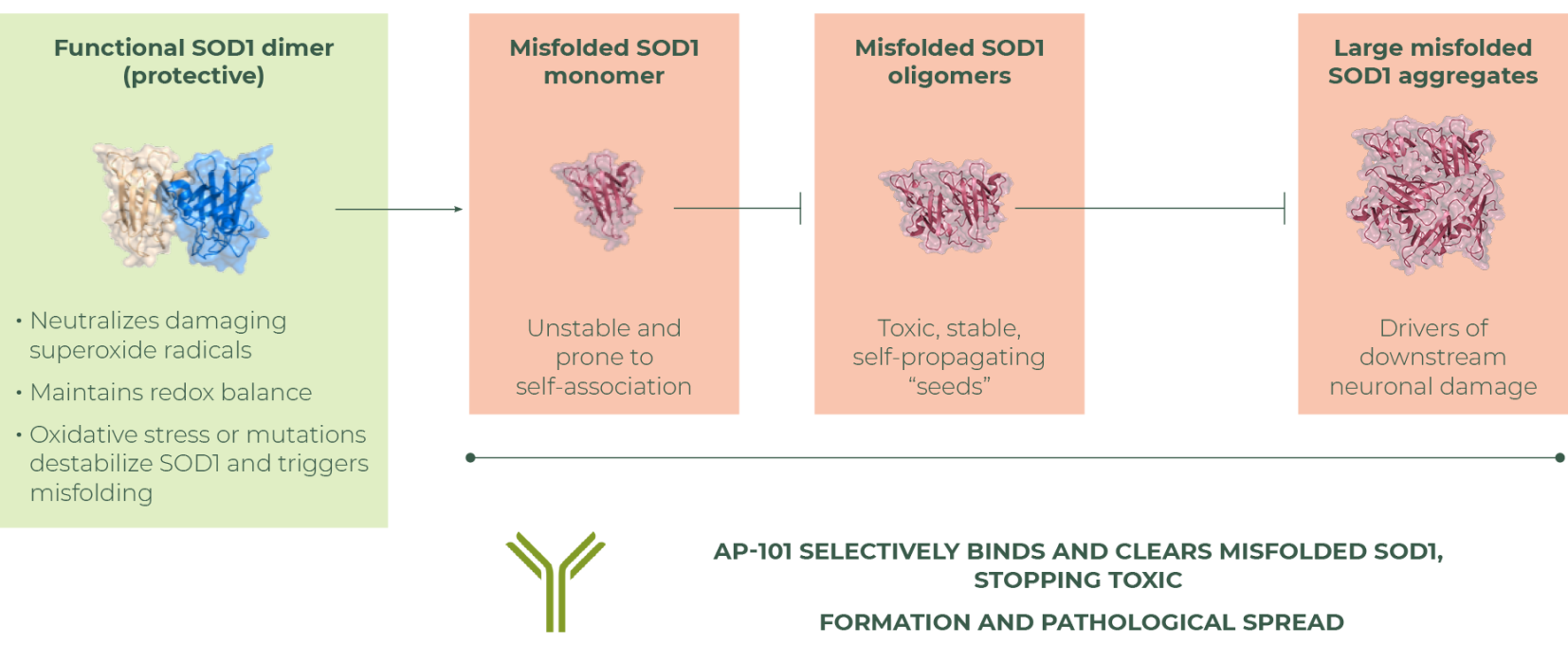
Despite this diversity, many patients share common downstream pathologies involving axonal injury, inflammation, and protein misfolding. SOD1 is a protective enzyme that helps cells manage oxidative stress. In ALS, structural changes can cause SOD1 to lose its proper function and misfold, taking on toxic conformations that disrupt cellular function. Such misfolded SOD1 can injure motor neurons, damage mitochondria and impair axonal transport. Pathology can spread by the seeding of SOD1 misfolding.
Misfolded SOD1 represents a powerful therapeutic opportunity. Across different presentations of ALS, misfolded SOD1 can amplify axonal injury and accelerate disease progression, making these toxic conformations an important target for intervention.
AP-101 reduces SOD1 pathology & extends survival in preclinical ALS models
Maier, et al.
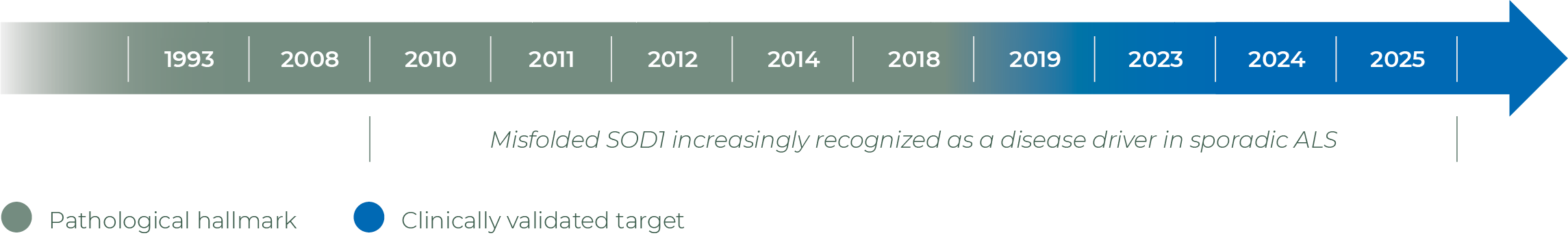
Initiation of AP-101 Phase 1 study in sporadic ALS & SOD1-ALS; Presence of misfolded SOD1 confirmed in sporadic ALS CSF samples
Tofersen has been approved by both the FDA and EMA for the treatment of SOD1-ALS specifically targeting the SOD1 gene mutation, with ongoing Phase 3 trials confirming its therapeutic benefit
Presence of SOD1 seeds confirmed in sporadic ALS, recombinant SOD1 mAb abolishes seeding in vitro
Mielke, et al.
The potential role of misfolded wild-type SOD1 protein in sporadic ALS: a review of evidence
Marlow, et al.
Topline Phase 2 AP-101 results show clinically meaningful changes related to survival and non-invasive ventilation in ALS patients
Initiation of AP-101 Phase 1 study in sporadic ALS & SOD1-ALS; Presence of misfolded SOD1 confirmed in sporadic ALS CSF samples
Tofersen has been approved by both the FDA and EMA for the treatment of SOD1-ALS specifically targeting the SOD1 gene mutation, with ongoing Phase 3 trials confirming its therapeutic benefit
Presence of SOD1 seeds confirmed in sporadic ALS, recombinant SOD1 mAb abolishes seeding in vitro
The potential role of misfolded wild-type SOD1 protein in sporadic ALS: a review of evidence
Topline Phase 2 AP-101 results show clinically meaningful changes related to survival and non-invasive ventilation in ALS patients

Science Translational Medicine – 2018
A human-derived antibody targets misfolded SOD1 and
ameliorates motor symptoms in mouse models of amyotrophic
lateral sclerosis.

«Our understanding of ALS has evolved significantly, and misfolded SOD1 species are now recognized as central drivers in patients with and without hereditary ALS. AP-101 represents a scientifically validated approach to target this pathology.»
Trial Investigator